Modern engines require a little more from today’s coolants.
The requirements of many vehicle owners on the coolant in a vehicle’s cooling system are extremely low. The coolant shouldn’t freeze in wintertime and it shouldn’t overheat in the summer months. That’s it. In truth, however, the requirements on coolant for state-of-the-art engines are extremely high, not least because even standard engines are becoming lighter in weight, more compact and more efficient with their components becoming hotter in the process. The increasing use of materials such as aluminium, magnesium and plastics also necessitates better and better wear protection due to the need to dissipate the heat that is generated while driving. This is one of the coolant’s main tasks but certainly not the only one.
Protection from frost and overheating
Almost every antifreeze (concentrate) is made up of around 90 percent glycol and 10 percent additives (also known as inhibitors in the case of antifreeze). Additives are supplements which affect the antifreeze’s properties. Car coolants are a mixture of water and antifreeze. The ideal mixing ratio is 1:1 which equates to frost protection down to -36°C. The maximum possible frost protection of approximately -52°C is achieved with a mixture of approximately 2:1 (antifreeze:water). Figure 1 shows the frost resistance of the water and antifreeze mixture as a function of the ratio.
Tip
Never use pure undiluted antifreeze as it will freeze at only -l6°C and also dissipates heat poorly. Water has a thermal conductivity that is approximately four times higher than glycol. More water in the mixture therefore results in better cooling. Pure antifreeze reduces the efficiency of cooling by around 50% compared to a 1:1 mixture.
In addition to lowering the freezing temperature, glycol also contributes to increasing the boiling point which protects the engine against overheating in warmer months. With a 1:1 mixture of water and antifreeze, the coolant’s boiling point is around lOre, thus providing the coolant system with a huge efficiency reserve.
The assertion that distilied water should be used in the cooling system is a hangover from the past when antifreeze containing phosphates were used. These phosphates were not compatible with the minerals in the tap water.
Tip
Now that antifreeze no longer contains any phosphates, there is no reason not to use normal tap water of drinking quality. If the tap water is extreme ly “hard”, a mixture of tap water and distilled water can also be used.
The basic advantage of distilied water is that it contains virtually no minerals that might be deposited in the engine. However, as it is perfectly possible to combine present-day antifreezes with comparatively hard water, this no longer presents a problem. By comparison, the disadvantage of distilled water is that it has a pH value lower than tap water. Pure, triple distilled water achieves a neutral pH value of 7.0. Tap water has slightly alkaline values, mostly around 7.5- 8.0.
The coolant in the engine should be slightly alkaline in any case (pH value >7) and under no circumstances should it be acidic (pH value <7) otherwise the gaskets come under attack. Additives which ensure that acids are bound and the coolant remains alkaline are added to every antifreeze for this reason. Acids are more likely to form if only distilled water with a lower pH value than tap water is used for the radiator mixture. The result is that the active substances in the antifreeze which are supposed to bind these acids are then used up more quickly. This can shorten the coolant’s useful life. So the low amounts of minerals in the tap water are of little consequence when compared to the distilled water’s poorer pH value.
Lubrication of the cooling system
Antifreezes additionally have lubricating properties enabling the coolant to be used as a lubricant for components of the cooling system (e.g. water pump, thermostat, heating valves). This is particularly important for the water pump’s mechanical shaft seal which would wear out after a short time without antifreeze (see Figure 2). Constant lubrication and cooling is necessary to guarantee that the mechanical shaft seal will function steadily throughout its service life. A small flow of coolant through the seal ensures that this is the case. The lubricating flow, which finds its way between rotating seal ring and stationary seal ring, is very small and can even evaporate in the pump. However, coolant can get into the free space behind the mechanical shaft seal and may escape at the drainage hole. The manufacturer factors this drainage in and it does not constitute any reason for complaint. If, however, an inferior coolant is used, rotating seal ring and stationary seal ring dry rub against each other without the protective lubricating film. This causes friction heat which can destroy the mechanical shaft seal.
Protection from corrosion
The inhibitors in the antifreeze also protect against corrosion and cavitation as well as preventing deposits and foaming. Silicate is an additive with excellent corrosion prevention properties. If the mixing ratio of antifreeze to water is calculated wrongly, the level of protective inhibitors in the coolant may be too low. It can lead to corrosion throughout the whole cooling system (see Figure 3). In this case, rust, limescale or dirt may destroy the surfaces of the mechanical shaft seal. As a result, the sealing of the water pump bearing is no longer guaranteed.
Tip
It is advisable to clean and flush the coolant system when replacing the coolant. Do not re use the coolant that is drained off. Coolant is hazardous waste!
Antifreeze by SWAG is safe from the weather and corrosion
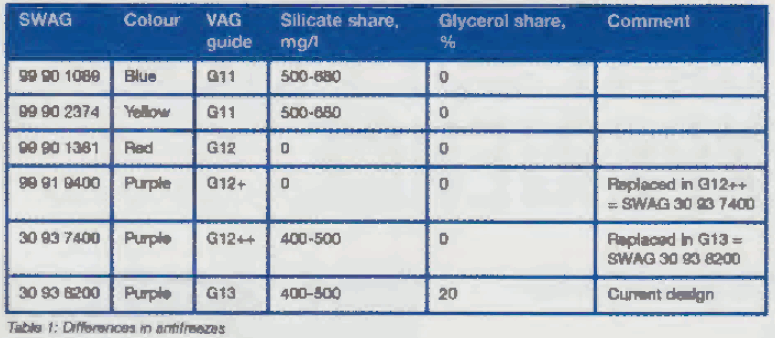
Silicate is now an indispensable additive given the increasing requirements in respect of material compatibility, corrosion protection , extended service intervals and the use of lighter weight materials in vehicle construct ion. However, the proportion in newer antifreezes has been reduced compared to older antifreezes with blue (cf. SWAG 99 90 1089) and yellow (cf. SWAG 99 90 2374 colouring.
Nevertheless, some manufacturers (e.g. BMW and Mercedes-Benz) are still using antifreezes with a higher proportion of silicate. Of the antifreezes available from SWAG, there are three that no longer differ in colour compared to their predecessors. The three antifreezes containing purple dye which are therefore visually identical (SWAG 99 91 9400, SWAG 30 93 7400 and SWAG 30 93 8200) have MEG (monoethylene glycol) as their base but exhibit differences in the percentage of additives (see Table 1).
The antifreeze in its current version now consists of approximately 70 percent glycol, 20 percent glycerol and 10 percent additives. Glycerol has similar properties to glycol but is more environmentally compatible and less energy is consumed by comparison during its manufacture. Corrosion protection and material compatibility have been further enhanced by new additives.
Mixability
Generally speaking, pay attention to the colour of antifreeze and always use antifreeze of the same colour in the vehicle. In spite of this, all SWAG antifreezes can be mixed with each other. The only exception is the red antifreeze (SWAG 9 90 1381) that must NOT be mixed with the olue (SWAG 99 90 1089) and yellow (SWAG 99 90 2374) products!
Service intervals
The coolant has a specific wear life. Over time, some of the inhibitors are used up. As a result, the coolant loses its frost and corrosion protection as well as its lubricating effect and thermal conductivity. Foaming and deposits may also occur. A coolant’s shelf life depends on its quality and the cleanliness of the overall cooling c;ystem. Wear is particularly intensive if a leak Jccurs or exhaust gases get into the cooling ystem (e.g. due to a faulty cylinder head gasket). It is therefore advisable to check the coolant regularly and replace it if necessary.
Tip
It is imperative to follow the manufacturer’s instructions regarding specifications, service intervals, mixability and mixing ratio.